

by Matthew A. Held, Ph.D.
Wed, Jan 15th, 2025 9:17 am
Leukemias are a relatively common set of blood cancers, with close to half a million cases diagnosed worldwide every year. An increase in the availability of diagnostic assays, and advances in leukemia treatments, have overall increased remission and survival time over the last several decades. Importantly, every diagnostic and follow-up laboratory test rely on the parallel usage of proper controls to assure cancer test results are accurate and precise.
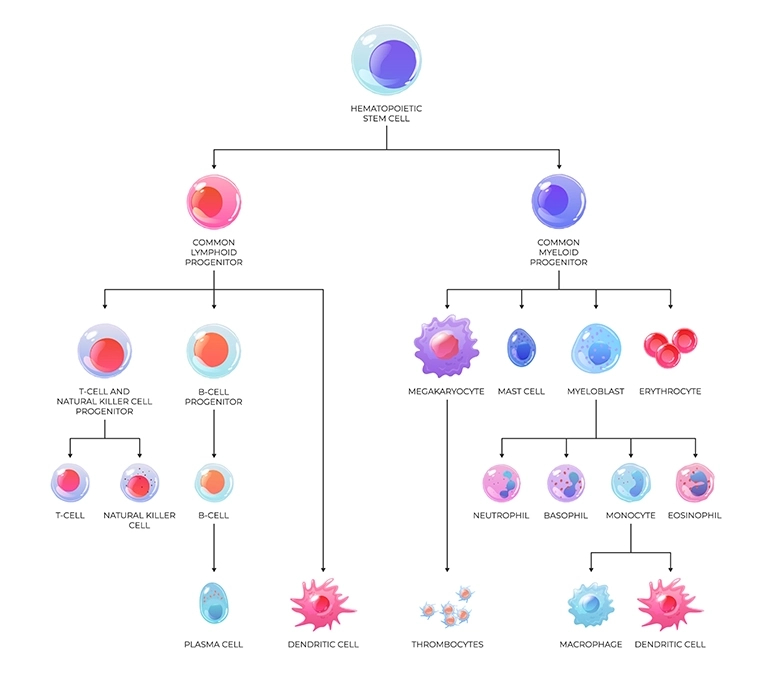
There are 2 main leukemia types depending on what cell type they originate from:
Additionally, there are 2 subtypes related to the rate of progression:
Together, this leads to 4 main types of leukemias:
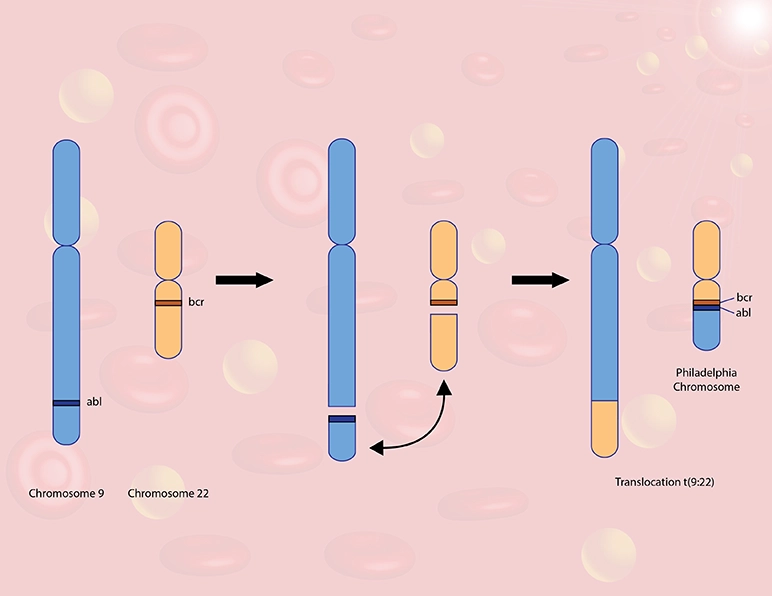
BCR-ABL1 is an abnormal fusion between a fragment of the BCR gene on chromosome 22 and a fragment of the ABL1 gene on chromosome 9. This translocation is found in the majority of CML patients, and up to 30% of ALL patients, but typically not seen in CLL or AML patients.
BCR-ABL1 is known as the ‘Philadelphia Chromosome’, as it was first identified by by Peter Nowell and David Hungerford at the Fox Chase Cancer Center in Philadelphia, PA in the 1960’s.
ABL1 is a critical gene that controls cell division and survival in early precursor myeloid and lymphoid cells. When fused with the BCR gene, it can lead to chronic activation of the ABL1 protein, resulting in a disruptive, life-threatening level of increased cell division.
There are 3 common types of BCR-ABL translocation mutations depending on where the breakpoints are:
Pharmacological studies performed by Dr. Brian Druker in collaboration with Ciba-Geigy (now Novartis) throughout the 1990’s led to the development of a ‘targeted’ cancer therapy known as Gleevec (imatinib), which inhibits the overactive ABL1 protein. The first phase I trials showed an astonishing 100% response rate, which was unprecedented for any leukemia drug. The ensuing success of Gleevec for treating CML patients was monumental: The drug improved the previous 5-year CML survival rate of ~20-30% up to ~80-90%, and has a high rate of preventing CML from becoming AML. The drug also works just as effectively for all forms of the BCRABL1 fusion CML cases, and for those majority of patients that respond well to Gleevec, life expectancy has now become close to normal life expectancy. Therefore, tests and controls aimed at identifying these BCR-ABL1 mutations are critically helpful in dictating therapy with Gleevec to maximize treatment efficacy.
Several diagnostic assays for the detection of BCR-ABL translocations are on the market today:
BCR-ABL translocation tests include:
Regulatory requirements mandate the use of External Quality Controls (EQCs) for ensuring the accuracy and reliability of results
You may be wondering : “Why are external controls even necessary to run with these leukemia assays? Don’t they already come with their own internal controls?” The answer to this lies in understanding the usefulness of a true external assay control, and what advantages it has over internal controls and kit controls.
Internal controls are part of the assay itself and do not go through the entire sample-to-answer process. They give information only about the performance of the internal components of the assay, and often are more forgiving with respect to calling an assay run ‘passing’. Likewise, some kit controls do not go through the entire test process and are typically provided for use solely with the associated kit components. Internal and kit controls are usually manufactured by the same company as the assay itself, therefore are not true independent controls. Together, the limitations of relying only on internal and kit controls include:
External Quality Controls (EQCs) go through the entire pre-processing part of the assay, and then throughout the entirety of the assay, just like a patient sample. EQCs are made by independent companies and specifically designed to inform on any failing reagents used in sample pre-processing steps, or on concerning trends of assay performance over time, across unique reagents lots, that may not be revealed reliably by internal controls or kit controls. As such, EQCs provide meaningful oversight that are manufactured to provide:
An additional consideration is the usefulness of an EQC that is also ‘quantitative’ in nature. Since standard leukemia testing is not just designed to determine a diagnosis, but to also identify how much of the associated biomarker is present for the purpose of directing therapeutic strategy, a quantitative EQC (qEQC) is critical and provides:
Well-designed qEQCs are especially important for providing high confidence in determining how well a leukemia patient’s treatment is working by assessing the presence of Measurable Residual Disease (MRD). For example, the MRD for a BCR-ABL1 positive CML patient is typically defined as when the BCR-ABL1 transcript levels are below 0.1% on the International Scale (%IS). Only qEQCs are manufactured in a way to provide the consistent reliability at and below the MRD in order to safely conclude that a patient is either in remission, or not.
In summary, quantitative EQCs:

Since the summer of 2021, Matthew A. Held, Ph.D. has been a scientist in the R&D department at Maine Molecular Quality Controls, Inc. (MMQCI) in Saco, Maine. He has been a Lead Project Scientist on many different control products for various diagnostic applications including FDA and CE- cleared oncology and infectious disease clinical lab assays, and is responsible for the development of these products throughout their entire life-cycle, from design to market launch.
Dr. Held earned his PhD in Cell & Molecular Biology at the University of Vermont, Robert Larner College of Medicine in 2009 and attained further postdoctoral training at Yale Medical School and Massachusetts General Hospital before joining Maine Medical Research Institute and the University of New Hampshire as a staff scientist. His academic research spans over 15 years in the fields of solid tissue oncology, and hematological development.

Matthew A. Held, Ph.D.