
Development and Validation of a Synthetic External Control Panel to Confirm Linear Dynamic Range and Quantitative Detection of PML-RARA
AMP 2024 — Vancouver, British Columbia Canada

Development of a Synthetic Secondary Standard for the Quantification of p210 BCR-ABL1 Standardized to the International Scale (IS)
AMP 2024 — Vancouver, British Columbia Canada
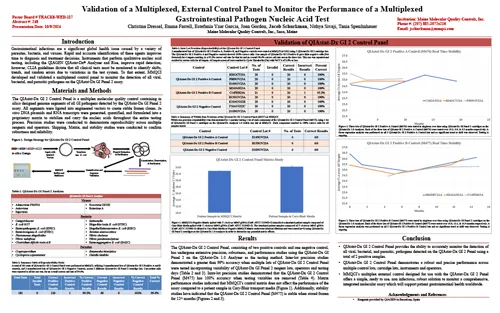
Validation of a Multiplexed, External Control Panel to Monitor the Performance of a Multiplexed Gastrointestinal Pathogen Nucleic Acid Test
CVS 2024 — Long Beach, CA USA
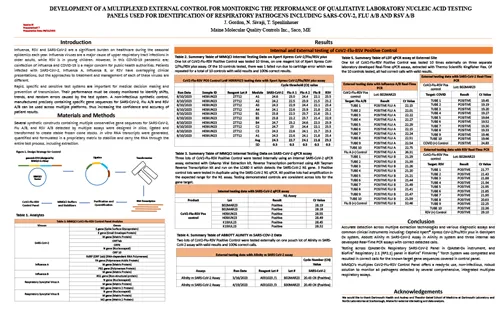
Development of a Multiplexed External Control for Monitoring the Performance of Qualitative Laboratory Nucleic Acid Testing Panels Used for Identification of Respiratory Pathogens Including SARS-CoV-2, Flu A/B, and RSV A/B
CVS 2023 — West Palm Beach, FL USA

Development of an External Control Panel to Monitor the Performance of a Multiplexed Gastrointestinal Pathogen Test
CVS 2023 — West Palm Beach, FL USA

Development of an External Control Panel to Confirm Linear Dynamic Range of Xpert NPM1 Mutation Assay
AMP 2023 — Salt Lake City, UT USA

Development of External Controls for SMA Carrier Screening to Accurately Monitor SMN1/SMN2 Copy Number Variation and Disease Modifier Variants
AMP 2023 — Salt Lake City, UT USA
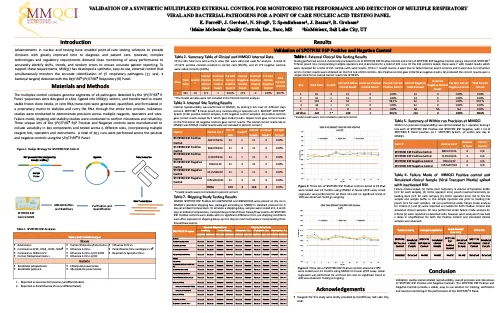
Validation of a Synthetic Multiplexed External Control for Monitoring the Performance and Detection of Multiple Respiratory Viral and Bacterial Pathogens for a Point of Care Nucleic Acid Testing Panel
IDWeek 2023 — Boston, MA USA
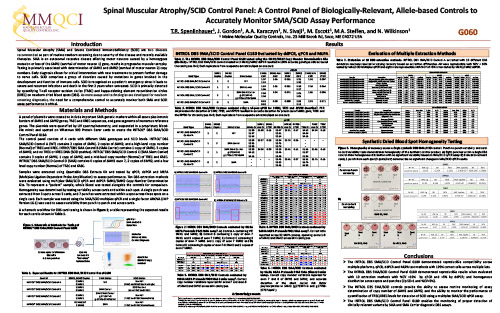
Spinal Muscular Atrophy/SCID Control Panel: A Control Panel of Biologically-Relevant, Allele-based Controls to Accurately Monitor SMA/SCID Assay Performance
AMP 2022 — Phoenix, AZ USA
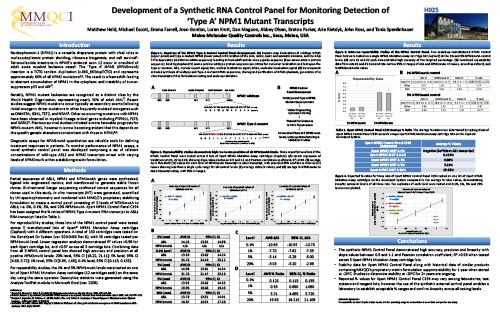
Development of a Synthetic RNA Control Panel for Monitoring Detection of ‘Type A’ NPM1 Mutant Transcripts
AMP 2022 — Phoenix, AZ USA
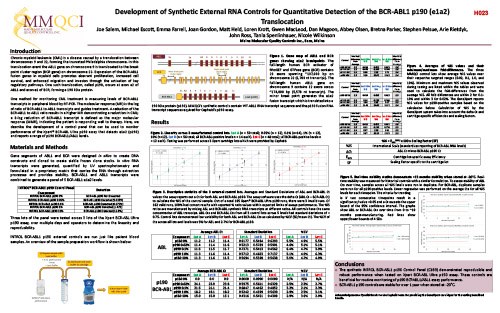
Development of Synthetic External RNA Controls for Quantitative Detection of the BCR-ABL1 p190 (e1a2) Translocation
AMP 2022 — Phoenix, AZ USA
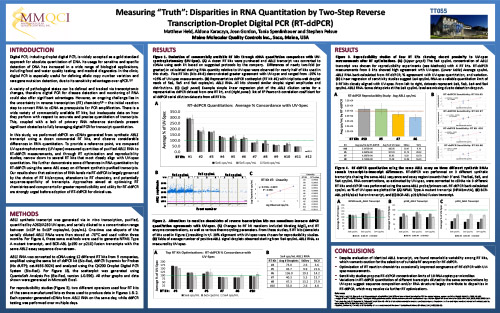
Measuring “Truth”: Disparities in RNA Quantitation by Two-Step Reverse Transcription-Droplet Digital PCR (RT-ddPCR)
AMP 2022 — Phoenix, AZ USA

Feasibility of a Synthetic Dried Blood Spot Mimic for use as an External Control for Newborn Screening of Genetic Disorders
AMP 2019 — Baltimore, MD USA

Development of Synthetic Multiplexed External Controls for Monitoring the Performance of Qualitative Laboratory Nucleic Acid Testing Panels Used for Identification of Lower Respiratory Pathogens
AMP 2019 — Baltimore, MD USA

Development of Synthetic Multiplexed External Controls for Monitoring the Performance of Qualitative Laboratory Nucleic Acid Testing Panels Used for Rapid Identification of Blood Culture Pathogens
CVS 2019 — Savannah, GA USA

Validation of a multiplexed synthetic control panel to monitor the performance of simultaneous detection of multiple respiratory viral, bacterial pathogens, and antimicrobial resistance genes in lower respiratory infections
CVS 2019 — Savannah, GA USA

Development of Synthetic Multiplexed External Controls for Monitoring the Performance of a Qualitative Laboratory Nucleic Acid Testing Panel Used for Rapid Identification of Respiratory Pathogens
CVS 2019 — Savannah, GA USA

Multiple Synthetic Reference Material for Monitoring the Analytical Performance of Highly Complex Variant Detection of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) using Next Generation Sequencing
AMP 2018 — San Antonio, TX USA

Development of synthetic secondary standards for BCR-ABL1 quantification on GeneXpert® BCR-ABL Monitor V2 and Xpert® BCR-ABL Ultra assays
AMP 2018 — San Antonio, TX USA

Utility of a Multiplex Synthetic Control Material for Monitoring the Identification of Multiple Variants of Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) using Next Generation Sequencing
AMP 2016 — Charlotte, NC USA