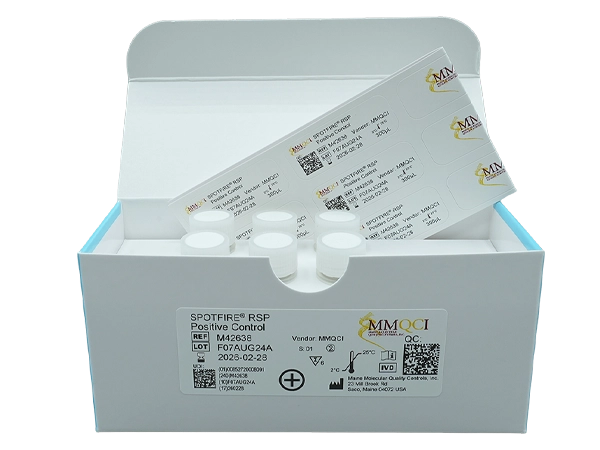
SPOTFIRE® RSP Positive Control
SPOTFIRE®RSP Positive Control is intended for use (as applicable) as an external positive assayed quality control to monitor performance of in vitro laboratory nucleic acid testing procedures for the qualitative detection of pathogens on the BIOFIRE® SPOTFIRE Respiratory (R) Panel, BIOFIRE SPOTFIRE Respiratory (R) Panel Mini, and BIOFIRE SPOTFIRE Respiratory/Sore Throat (R/ST) Panel on the BIOFIRE SPOTFIRE System.
Product Information:
SPOTFIRE® RSP Positive Control
Part Number: M42638
GTIN: 00852720008091
Kit Contains: 6 tubes x 300µL
Controls/Qty: 6 x Positive
Storage: Room temperature (18° – 25°C) or refrigerated (2° – 8°C)
Documents:
You might be interested in: