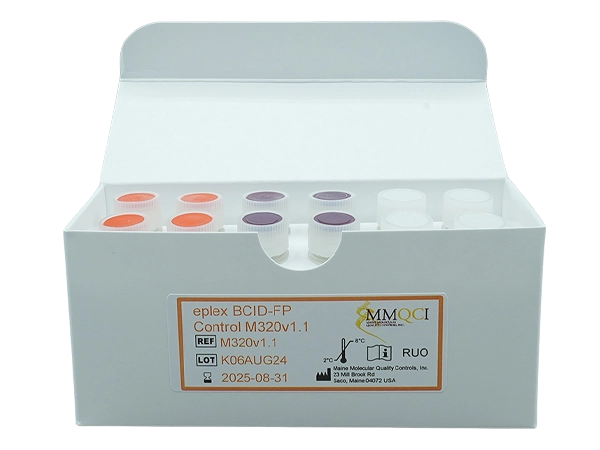
eplex BCID-FP Control M320v1.1
The eplex BCID-FP Control M320v1.1 is intended for in vitro use as a qualitative external quality control to monitor the amplification, detection and identification steps on the cobas® eplex blood culture identification fungal pathogen (BCID-FP) panel performed on the cobas eplex system (Roche Diagnostics).
Product Information:
eplex BCID-FP Control M320v1.1
Part Number: M320v1.1
GTIN: Not available at this time
Kit Contains: 12 tubes x 50μL
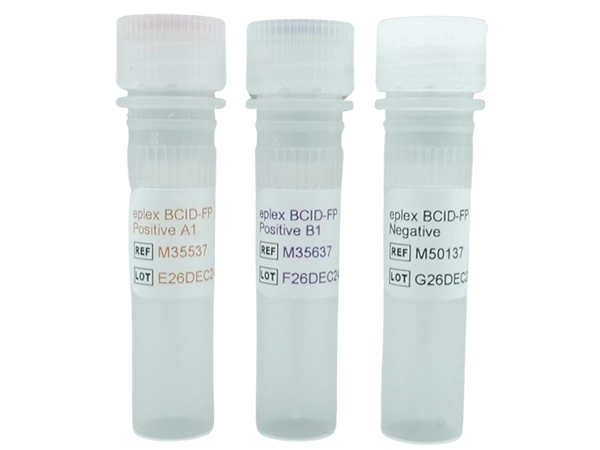
Controls/Qty: 4 x Positive A1 M35537, 4 x Positive B1 M35637, and 4 x Negative M50137
Storage: Refrigerated (2° – 8°C)
Documents:
You might be interested in: